With over 400,000 women implanted with the PIP devices, there is understandable concern. For those women that were implanted with PIP breast implants, how significant is the rupture rate and should they be replaced even if they are not ruptured? There is no general consensus on either issue. Previous reports have indicated that PIP breast implant rupture rates are fairly low, being less than 5% after a decade of implantation.
More recent studies, however, have indicated the failure rates are much higher. A recent report from the UK suggests that the failure rates for PIP breast implants may be as significant as one in three patients over a decade or more after having been placed. The higher detection rate, as admitted by the authors of the study, is due to more detailed evaluations through ultrasounds rather than just physical examination alone.
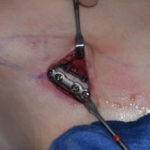
Under normal conditions, even when a breast implant ruptures the material is self-contained. The layer of scar that naturally forms around a breast implant is significant and does not allow what is inside to get outside. It is the body’s clever way of living with foreign materials by establishing a boundary, known as the capsule, between one’s natural tissues and the implant. Thus ruptured breast implant contents remain confined inside a capsule ‘prison’ whose innermost lining is avascular and relatively impermeable to large polymerized silicone molecules.
But the problem with the ill-manufactured PIP implants is that the silicone filler was non-medical grade. What other materials or byproducts would have been in the silicone gel is unknown. How this affects the potential diffusion or transport of loose silicone gel beyond the capsular lining is unknown. As a result, all ruptured PIP implants certainly need to be removed as soon as possible.
All PIP-implanted women need to undergo diagnostic testing to determine if they are ruptured and, if not, should be monitored annually. Based on this most recent information of higher than expected rupture rates, it is likely that within the next ten years most PIP breast implants will have been removed and replaced.
Dr. Barry Eppley
Indianapolis, Indiana