The removal of fat without liposuction has led to a wide variety of non-surgical treatment approaches. These include numerous energy-based treatments which are applied externally. While one can debate the merits of the various devices and their technologies, they all have limits in terms of effectiveness and the speed at which results are seen. This has led to the pursuit of other non-surgical approaches.
Like Botox and fillers an injectable approach is a cross between non-invasive treatments and invasive surgery like liposuction. There is prior history of using injections for fat reduction and it dates back to a decade ago when the concept of Lipodissolve was very popular. At that time mixtures of phosphatidyl choline (PPC) and deoxycholic acid (DCA) were used in wide scale clinical use in clinics throughout the U.S. for fat reduction all over the body. Due to a variety of reasons, financial and lack of an FDA studied and approved agent, Lipodissolve literally ‘dissolved’. But the concept remained that some fat reduction could be achieved by injection although it was unclear what the active agent actually was. (PPC vs DCA)

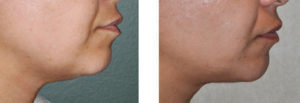
Patients received up to 6 treatments (grid delivered microinjections) spaced one month apart. At three months after the last treatment, almost 90% of the patients achieved a visible improvement. Patients said they felt less bothered by their double chin, were less self-conscious, and felt they looked like they had lost weight. Almost all patients experienced no skin laxity in the neck as the fat was lost. Laboratory studies showed no changes in the patient’s blood lipid levels as the fat was broken down. Almost every patient experienced swelling and some minoir bruising for up to a week after the injection, which is a well known immediate and temporary effect of this subcutaneous injection agent.
Dr. Barry Eppley
Indianapolis, Indiana


