Although Botox has been used for decades to treat facials wrinkles, it was only formally approved by the FDA in 2002 for the temporary improvement of one very specific type of facial wrinkle, the glabellar lines between the eyebrows. This was what it was studied for in clinical trials and that is what the manufacturer is limited to in requesting FDA approval. That is what it says on the product packaging and that is what the company can say it does in any marketing and advertising pieces. This is how the FDA understandably works.
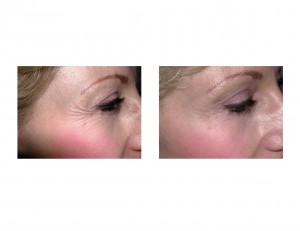
Now that Botox has been approved for the treatment of crow’s feet, it will not likely change how doctors employ Botox in their practice or in the number of patients being treated. But it will change how the manufacturer markets Botox and in the advertisements you see. Expect to see a big push and awareness of the crow’s feet problem and how it can be effectively treated. This will be particularly so since Botox’s competitors (Dysport and Xeomin) do not yet have this same approval.
Out of the over 800 patients that had crow’s feet treated in the clinical trial submitted for FDA approval, the most common side effect was some temporary swelling of the eyelids. This is interesting because that is a temporary side effect that I have never seen. I have seen some bruising due to large hidden veins that run through the crow’s feet area that get nicked from the needle but never eyelid edema.
But as for effectiveness, the reduction or eradication of crow’s feet occurs just as predictably as Botox injections do for the glabellar lines.
Dr. Barry Eppley
Indianapolis, Indiana


