Background: The use of silicone implants for breast augmentation has now been done since 2006 when this implant filler material was re-introduced as an implant option. One of the stated restrictions with their use was that patients had to be 22 years or older. This was a position imposed on the manufacturers as issued by the FDA. As a result this is on the package inserts as indications for clinical use.
This silicone breast implant guideline from the FDA causes considerable confusion. Many breast augmentation patients under 22 years old want silicone rather than saline implants but feel they can not have them. Plastic surgeons may also follow this guideline rigidly feeling that they don’t want to be violation with the FDA. They may also feel that implanting young patients with these devices may invalidate the manufacturer’s warranty.
There is some debate as to why the FDA imposed this clinical guideline. One belief is that there were no patients under 22 years old in the submitted clinical study used for FDA evaluation. This would seem to be the most logical explanation but apparently there some such patients in the study. It is more likely that there was simply not enough patients in this age group to draw the same conclusions as the older patients.
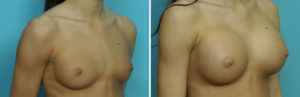
Case Study: This 18 year-old female wanted breast implants but preferred silicone over a saline implant fill. She developed little natural breast tissue and had small but visible breast mounds.
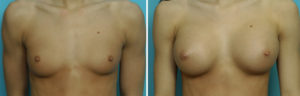
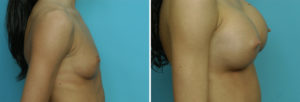
There is no clinical difference in the use of silicone breast implants whether one is older or younger than the age of 22 years old. Their use should be based on surgeon discretion and presurgical patient education.
Highlights:
- Per manufacturer guidelines, silicone breast augmentation is for patients 22 years and older.
- Silicone breast augmentation can be done on women as young as age 18.
- The tight skin of young patients will create more of a round breast look with implant placement.
Dr. Barry Eppley
Indianapolis, Indiana