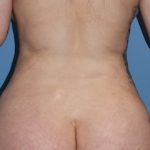
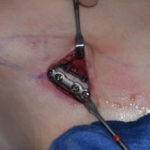
Injectable fillers are the other half of injection treatments (Botox is the other half) that have revolutioned the approach to facial. Evolving from the first FDA-approved injectable filler in 2002 (Restylane), there are now over a dozen different facial fillers from various manufacturers. The latest filler options have become focused on filling a specific type of facial volume indication which is not a surprise as the filler field has become crowded and new product introductions must fill a specific niche to become a used option.
Juvederm Voluma XC is made with the proprietary Vycross technology that allows the gel to be injected smoothly and consistently through a needle or microcannula. Like other Juvederm products, it also contains lidocaine which provides a numbing effect during the treatment. Juvederm Voluma XC is not new, it has been used in Europe since 2005 without lidocaine and since 2009 with lidocaine in it. The potential side effects with its use are exactly like that of any filler, temporary swelling, bruising, and lumps and irregularities.
Juvderm Voluma XC is a hyaluronic-acid injectable filler that can now rival other fillers used for their facial volumizing effects such as Sculptra and Radiesse.
Dr. Barry Eppley
Indianapolis, Indiana