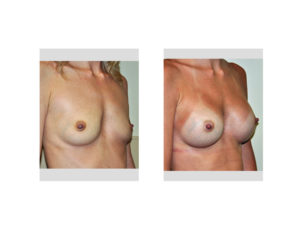
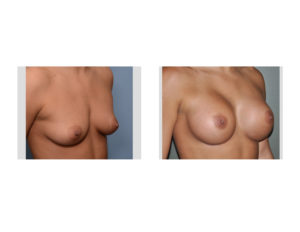
The most common reason any implant infection occurs is bacterial contamination and inadvertent inoculation of the implant. While the implant may be sterile in the packaging there are numerous opportunities for it to become inoculated between the box and the implant pocket. While most plastic surgeons use numerous safeguards to prevent infection there are no standards of practice amongst all of them.
In the June 2017 issue of the Annals of Plastic Surgery, an article was published entitled ‘Antimicrobial Prophylaxis Practice Patterns in Breast Augmentation: A National Survey of Current Practice’. In this paper, a surgery was sent to members of the American Society of Plastic Surgeons to assess their practice patterns of preventing infections in breast augmentation surgery. Of all the members solicited just over 250 responses were obtained. The results of the surgery showed that Chlorhexidine was used for surgery site prep in just about 50% of the respondents and a triple antibiotic solution was used for both implants soak (40%) and pocket irrigation (almost 50%) before implant placement. Interestingly over 40% of the surgeons used a no-touch funnel for implant insertion. After surgery antibiotics included a first-generation cephalosporin (Keflex) in almost 80% of respondents and was used up to one week after surgery in about half of the reported surgeon’s practices.

Dr. Barry Eppley
Indianapolis, Indiana


