Since silicone implants were re-introduced eight years ago in 2006, they have gradually returned to their dominance over saline breast implants. With a more natural feel, lack of a deflation risk and being longer lasting, they have numerous advantages over saline-filled devices. But despite their commercial availability they are not available to all women who desire breast augmentation. By the manufacturer’s guidelines they are restricted to women who are at least 22 years or older.
This silicone breast implant restriction runs into a frequently posed question by younger patients…why can I not have these type of breast implants if I am younger than 22 years old? What could possibly be the difference between the breasts of a 20 year old vs a 22 year old? Why would saline implant be allowed but not silicone implants.
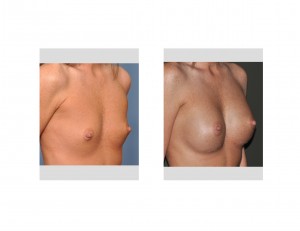
While this age restriction exists, some plastic surgeons do provide silicone breast implants to women under 22 years old. They take the position that this merely represents off-label use and within the province of the physician’s discretion and judgment. Conversely, many other plastic surgeons will not place silicone implants in these younger women as a strict adherence to the regulatory stipulations. Which one is right?
The answer is simply unknown. It is ultimately up to the plastic surgeon and what they feel in is the best interest of their patients and what their liability exposure is. At the least, patients under 22 years of age need to know of the manufacturer’s stated age restrictions and whether placing them at this age may affect the manufacturer’s warranties of the implants. (lifelong implant replacements) It is not clear what position the manufacturer’s take on this issue.
Dr. Barry Eppley
Indianapolis, Indiana


