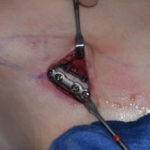
It has been shown in numerous experimental studies that fat graft survival and the volume retention that results is from the number of fat cells (adipocytes) surviving the transplantation process. While accompanying stem cells and other companion cells may have a contributing role in creating some of the augmentative effect (preadipocyte conversion), this cellular event is more minor than often touted. Thus harvesting and preparation of fat grafts must focus on how best to ensure the that the fat cells, hardy as they may be, are not extensively damaged in the process.
The absolute best methods to handle fat grafts are both multifactorial and incompletely understood. But considerable effort from experimental studies to surgeon’s anectodal and clinical experiences are beginning to unravel the onion layers of the process. The important steps include donor site selection, local anesthetic infiltration, harvesting cannula size and pressures, transfer exposure, and graft concentration techniques.
The least understood of these factors is the influence of the donor site which may eventually turnout to be one of the most important. Subcutaneous fat is now known to have subtle biologic behavior differences. Abdominal fat is different, for example, from inner knee or neck fat. as evidenced by how it responds to weight gain and loss. Whether this makes it more or less hardy is speculative. On a practical basis, the donor site is often driven by the volume needed. Facial fat grafting can be more selective in choosing a donor site, most body augmentations (breast and buttocks) can not.
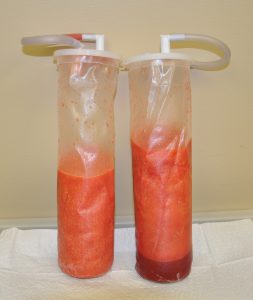
Ideally fat should be harvested by low pressure vacuum or syringe aspiration to prevent extensive disruption of fat cell walls by extreme differences in intra- vs extracelluar pressures. While negative 1 atmosphere (-20cms H20) is a common vacuum pressure in standard liposuction extraction, lower pressures should be used in harvesting reducing them closer to half that commonly used in fat removal that is going to be discarded.
In harvesting fat, small cannulas are usually preferred. Not because they traumatize the fat less, but because they reduce the fat particulate to a size that is more amenable to smoother injection through small cannulas later.
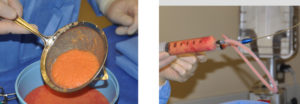
While many of these fat harvesting devices have their merits, when it comes to cost, efficiency of time and material management, I have taken a de-evolutionary step. I have evolved back to low tech simple bronchial washing traps for small harvests and a sterile glass jar for very large harvests. There use does make them an open system for transfer but the potential risk for contamination seems to be very low and not a clinical problem that I have seen in terms of infection.
Dr. Barry Eppley
Indianapolis, Indiana