Short of metal implants used for fixation and repair in bone surgery, most implants used in plastic surgery are composed of a silicone-based material. It may have varying states of being a solid, (soft to more firm) but silicone-containing implants have long been recognized as one of, if not the most, biocompatible synthetic material in existence. The breast implant fiasco in the early 1990s created a vast patient scare and its negative connotations still reverberate today. This is despite the fact that silicone breast implants received complete vindication as being harmful and were re-introduced for clinical use again in 2006.
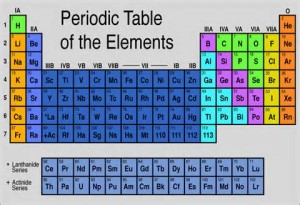
Silicon sits as a chemical element five vertical rows from the left and three horizontal rows from the top. It has the symbol Si and has an atomic weight of 14. It is what is called a tetravalent metalloid, which sounds like it is really a metal, although the term means that it has properties of both metals and non-metals. Joining Silicon as a metalloid are some familiar names from the very friendly Carbon (the basis of all organic life) to the very poisonous Arsenic. It is the second most common element available in the earth’s crust after oxygen, appearing in dust and sands usually in the form of silicon dioxide. (silica) It does not exist much in its purest form, but its use in that regard impacts all modern technologies as it serves as the basis of semiconductor electronics and integrated circuits.
Silicon has long served as the backbone for silicon-based polymers known as silicones. One should not confuse, however, Silicon and Silicone. The polymer Silicone does contain Silicon but it is put together with other elements such as oxygen and hydrogen which give it very different physical and chemical properties than elemental Silicon. These formulations create common products with a wide range of physical forms (soft to hard) such as silicone oils, rubber, caulk and a diverse number of medical implants. Silicone polymers have a large number of very favorable properties as an implanted material including remarkable stability (does not change over a temperature range of -100 to 250 degrees C), does not absorb water or other fluids, has little chemical reactivity, little known toxicity and does not support bacterial growth. Thus it is a structurally stable polymeric material that is not likely to degrade in any way over a patient’s lifetime.
The biocompatibility of a long-term implantable medical device refers to its ability to perform its intended function without creating any undesirable local or generalized effects. A silicone polymer fulfills that role well and, when combined with the wide availability and low cost of its base material, it is no wonder that most non-metal medical implants are made of some or all of it. Its easy moldability makes it able to be molded into almost any shape or size such as silicone gel breast implant, a soft solid pectoral or buttock implant and a soft but more firm facial implant.
But besides its unique physical properties when made into a polymer, is there anything else that makes it so biocompatible? It probably does not hurt that its closest vertical neighbor is Carbon. By its electronic composition, Carbon and Silicon are closely related event though they are distinct elements that form distinct compounds. But being next to the element that is responsible for all life on earth probably does not hurt how that life sees it.
Dr. Barry Eppley
Indianapolis, Indiana


