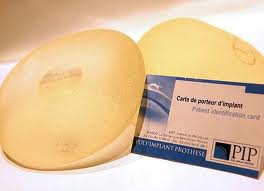
Europe is now experiencing its own breast implant scandal. The French-manufactured Poly Implant Prostheses (PIP) breast implant has been discovered to be manufactured with industrial-grade rather than medical-grade silicone material. This has resulted in the few months since this story has emerged in the company going defunct, its top executive arrested and a ground swell of concern amongst implanted patients. The PIP implant, which has been implanted in somewhere up to 50,000 European women, has a much higher than normal rupture rate. Whether this increases a woman’s risk of breast cancer and other diseases is not known.
Just like what happened in 1991 in the U.S., European countries are providing various and differing opinions as to implant patients should do… and who should pay for it. Some countries are recommending the removal of PIP implants as soon as possible due to their rupture risk while other countries are not recommending removal or have no opinion at all. Plastic surgery societies, such as the International Society of Aesthetic Plastic Surgery, and even the World Health Organization have proferred recommendations.
It is clear that there is no definitive opinion as what exactly should be done with implanted and asymptomatic PIP breast implants. There simply is not enough medical evidence to gauge what health risks, if any, that they pose. While this is a bigger concern in Europe, it is also of occasional interest for some U.S. patients. Some women have traveled abroad to have breast augmentation and some PIP implants have been imported (illegally) and inserted by U.S. surgeons. I know this to be true as I have removed PIP breast implants in two patients over the past three years for cosmetic and rupture issues.
What should existing PIP breast implant patients do? If you have any breast symptoms such as pain, hardness or nodules or lumps of the breast, get a mammogram or an MRI if necessary to determine if the implant(s) have ruptured. If a rupture exists then they should be removed and replaced as soon as possible. If your breasts feel fine and you have not had a mammogram in the past year, then you should get one to rule out silent rupture and establish a baseline for future imaging studies. If the implants are intact and you are comfortable with leaving them in place, then annual mammograms can be done for periodic surveillance. Even with intact indwelling PIP implants, it may be psychologically better for some patients to have them removed and replaced to end any concerns.
While this European breast implant scandal will not rise to the magnitude of what the U.S. experienced twenty years ago, there are some lessons to be transferred from that experience. While understandable concern and well intentioned opinions will abound, PIP breast implants should be diagnosed and treated like any other breast implant. Keep a high index of suspicion for implant rupture and replace when a rupture is detected. Explantation and/or replacement of intact implants is done if the patient’s concern so justify.
Dr. Barry Eppley
Indianapolis, Indiana