Injectable aesthetic facial treatments, since 2002, have continued to be developed and are now part of mainstream beauty treatments for many. Whether it is Botox, Dysport, or the variety of injectable fillers, their delivery is exclusively by injection with the attendant discomfort and apprehension that comes from needles in the skin. It would be appealing in my Indianapolis plastic surgery practice if some of these treatments could be topical or non-injection to bypass one of their few major drawbacks.
The concept of a topical neuromuscular blocker, or topical Botox, has been falsely advertised by numerous products over the past few years suggesting that they have similar effects. Their best advantage has been that they have played on the gullible and the hopeful. No real such topical product is commercially available at present. There are certain peptides that are part of some topical products that do have a very mild wrinkle-relaxing effect but it is nowhere near what Botox and Dysport can achieve.
There is a potential product under development, however, that does appear to be fairly promising. Phase II FDA-sponsored clinical trial reports from earlier this year have demonstrated some very positive effects with a topical product (Revance, CA) on the crow’s feet area. Phase II trials are a test of concept on a limited number of patients. The results were promising and they are currently beginning more extensive Phase III trials. Using a proprietary transdermal delivery system, it is possible to get a botulinum molecule past the skin’s surface. Given the thin skin of the lateral orbital area, this is likely to be its primary facial application. Whether it will be an adjunct to Botox and Dysport or an eventual substitute remains to be seen.
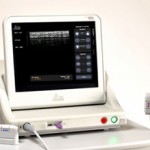
Somewhat similarly, a topical ultrasound device company has recently received FDA clearance for eyebrow lifts. Five year clinical studies for the Ulthera system showed that it had no serious side effects and essentially no recovery time with treatment times of around 30 minutes. It delivers ultrasound energy to reach tissues beneath the skin to induce collagen growth and gradual firming, tightening, and actual lifting of skin after a series of treatments. Its clinical effects result in less hooding and laxity of the eyelid skin and a more open look around the eyes which was judged to be significant. Other areas of the face also exhibit firmer and tighter skin following treatments. While other skin tightening and firming treatments currently exist, using different energy sources, Ultherapy uses focused ultrasound and may have more specific tissue level effects.
We can see that technologies for delivering non-injectable cosmetic treatments, with tangible clinical benefits, continues to be developed. This next wave of therapies will no doubt find their way as part of the growing managere of facial treatment options. They are sure to complement existing injectable therapies as well as postoperative maintenance approaches.
Dr. Barry Eppley
Indianapolis, Indiana