Augmenting facial and skull bones can be done by a variety of alloplastic materials. These biomaterials separate into two basic categories, preformed implants (e.g., solid silicone, Medpor, ePTFE) and bone cements. For both the face and the skull the vast majority of aesthetic augmentations are done by preformed implants whose value has become enhanced by contemporary computer 3D designing technology. Bone cements are far less used than implants because they require greater technical experience to successfully use and require larger incisions to be accurately placed and molded.
But for a select few patients the biologic appeal of bone cements sounds better as they appear to be more biocompatible and are not a true ‘implant’. To provide clarity on that issue let us put aside the challenges that come from using bone cements as an onlay skull and facial augmentation material and focus on their chemistry and biologic behavior.
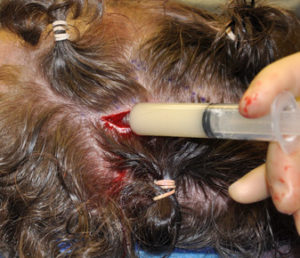
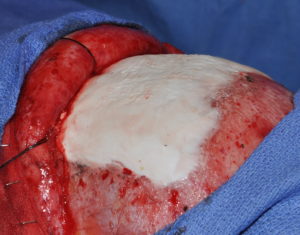
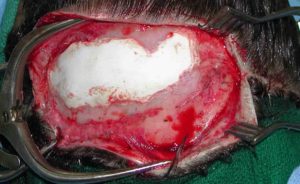
Hydroxyapatite cement preparation Dr Barry Eppley IndianapolisWhile HAC cements have better biocompatibility with bone than does PMMA bone cements, its working properties are substantially inferior. While both materials become putty-like when mixed with their respective monomers, they have very different flow properties which has major implications for how they are placed, shaped and set. Unlike PMMA which has a cohesive and smooth flow behavior, HACs do not have similar favorable handling properties. While they look a bit like Crisco shortening when mixed they have poor flow characteristics and must be pushed into place or a shape by hand. They are also sensitive to moisture (blood) which affects their shape and setting. (unlike PMMA which is completely resistant to such influences) These characteristics are why trying to place and shape HACs through limited incisional exposure is very challenging and the desired shape is not likely to be achieved. For these reasons HACs have their greatest use in a wide open surgical field in which it is placed into a defect that has walls to contain and shape it.
While PMMA can be accurately called a bone cement, HACs should not be. It was never developed to be a cement nor does it have the physical properties to do so. It should more accurately be called a synthetic bone substitute or replacement material. Its handling properties were developed to be placed into a well defined bone defect whose walls can contain and shape the material as it sets. Trying to use it as an onlay material (expanding the bone contours) in the skull and face requires great experience in working with it to create the desired augmentation shape.
Dr. Barry Eppley
Indianapolis, Indiana