Cranioplasty is done for making contouring changes to the cranial vault, which is defined as the skull, forehead and brow bones. Common causes for the need for cranioplasty are congenital deformities (after primary reconstruction), neurosurgical bone flaps, and traumatic injuries. When done for these reasons an open approach is always used as there is usually a pre-existing scalp scar/incision from a prior procedure. This makes it very easy to apply the traditional synthetic cranioplasty materials such as PMMA (acrylic) and HA. (hydroxyapatite)
However, some skull shape problems may be relatively small or may not be associated with any pre-existing incision for access. When balancing the trade-off of a new scalp scar versus keeping the existing skull concern, many patients (particularly men) would consider the scar as more undesireable. Cranioplasty would be more appealing in this circumstance if the cranioplasty material would be able to be delivered from small and remote incisions. In essence, a cranioplasty material that could be delivered by an injection process.
The current craniplasty materials are far from ideal to be delivered through any form of remote access. PMMA, polymethylmethacrylate, is an initial liquid which can be delivered through a tube but it is very runny on delivery and sets up with a very high heat from an exothermic reaction. The numerous forms of hydroxyapatite (e.g., Mimx) create an initial viscous slurry which has no material flow at all. This makes it not only undeliverable by injection but its sensitivity to fluids and its easy fragmentation on setting make external digital molding unpredictable.

Once the three ingredients are mixed, a very flowable liquid is created. During the intial polymerization process (up to 4 minutes), the material can be loaded into a syringe. Once in the syringe, it remains in a thick but flowable liquid phase up to 8 minutes are mixing. This provides the opportunity for delivery by injection. Once it passes the 8 minute time period, it enters a sticky taffy phase where it becomes very adhesive and is no longer injectable. A moldable phase will exist then up to 25 minutes in which further shaping can be done.
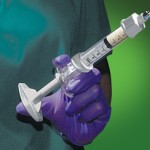
The other injectable consideration is can the material be effectively molded by external manipulation. Is it able to be pushed around and molded into fine edges without fracture or separation of the material? In testing on a pig’s head (from the butcher), Kryptonite was injected and externally molded to the back of the skull from an anterior incision. On dissection after setting, it was adherent to the bone, did not stick to the overlying soft tissue and had nicely contoured edges. Its sticky taffy phase which is between 5 and 15 minutes after mixing gives it a texture which really molds and shapes well.
Kryptonite bone cement appears to offer physical properties that would make it the first truly injectable cranioplasty material. Its use in this manner is for partial-thickness contour deformities of the skull and forehead.
Dr. Barry Eppley
Indianapolis, Indiana


