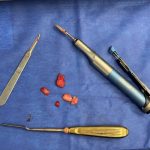
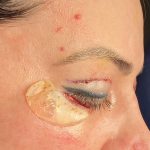
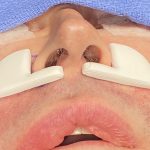
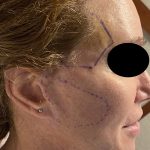
Plastic Surgery Case Study – Overlay Implant Augmentation After Custom Cheek Implants
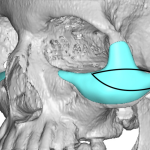
Background: Optimal augmentation around the orbit and cheeks is ideally done with a custom implant design. Some aesthetic cheek augmentations need a significant orbital component, most commonly along the infraorbital rim but may also include the lateral orbital rim as well. Often the desire for such an implant design is learned from the use of Read More…